The Care Metabolic Protocol, synergic combination of target metabolic drugs as additional therapy to fight Glioblastoma Multiforme
Currently, around 2,000 drugs have received approval from various agencies around the world. Most of these drugs fall into the category of small molecules (<900 dalton) and as such have a high degree of pleiotropy, thus modulating more than one path within the cell. This results in approximately 6 molecularly relevant targets per drug on average. Simple arithmetic reveals that the number of FDA-approved indications per drug does not reflect the quantitative opportunity that exists in the current pharmacopoeia. What this means, in short, is that there is a lot of unrealized potential within our existing drug pool. Occasionally, a clinically relevant “out of target” emerges by chance, giving the manufacturer the opportunity to “Reuse” the drug for the new indication observed.
A famous case is that of Viagra® which comes from a phase 1 study of Pfizer’s promising new compound labeled UK-92,480 for angina. The compound failed to relieve angina, but an interesting different off target effect unequivocally appeared in the patient.
In fact, however, there is a bias towards novelty and in fact pharmaceutical companies and academics are rewarded for innovation and therefore for inventing new drugs, although often, after the initial enthusiasm for the laboratory effects of a new molecule, its final clinical usefulness, which can only be reached after a long journey, can be disappointing. It is important to consider how many opportunities are lost in the frantic search for the latest news without taking full advantage of what we already have.
Discovering off-target effects from old drugs is the easy part: translating the effect into clinical operation, however, has proven extraordinarily difficult. Once a drug is no longer patented, there is no financial incentive for a pharmaceutical company to introduce it through the necessary tests to reuse it for a new indication. In fact, it is almost never done without first slightly changing the composition of the compounds or the method of combining the compounds in order to secure a new patent. The cost simply could never be recovered, leaving many generics as “financial orphans.”
The pharmaceutical industry may care little about the clinically relevant off-target effects of older drugs, but academia and nonprofits have a history of massive investment in research into the therapeutic effects of non-patentable compounds. Academics are constantly looking for interesting leads who have a high probability of being funded and, once identified, they tend to accelerate the search to quickly reach the result.
For example, the type 2 diabetes drug, metformin, was discovered to have anticancer activity in hamsters in 2001. This has led to a series of prospective population studies that have shown that type 2 diabetics treated with metformin receive statistically diagnosed with different types of cancer less frequently than the population average, and when diagnosed, they tend to respond better to treatments and live longer. However, the huge archive of data documenting metformin, statins and the anticancer activity of other generics has not been significantly translated into the clinic. The use of generic drugs repurposed for other clinical indications always lacks a randomized, placebo-controlled Phase 3 study (RCT) necessary for the FDA to stamp approval for a new indication.
And yes, the advantages of using repurposed drugs would be significant: price, safety (from a wealth of information on pharmacokinetics, dosage, and toxicity resulting from long-term use) and the ability to use multiple agents simultaneously (as they are known drug-drug interactions and drug-drug synergies).
The combination of mono-agents is a pharmaceutical strategy that has historically demonstrated far superior results in the treatment of complex diseases. The evolution of HIV treatment still today offers a lesson on the power of rationally designed combinations, or “cocktail” therapies. The Care Oncology Clinic, after carefully considering various data demonstrating the antitumor activity of the individual agents and, above all, the synergistic action between the compounds, in order to attack the cancer cell on several fronts, has selected a combination of 4 generic drugs with the best potential as an add-on cancer treatment he called the Care Metabolic Protocol ™. The four drugs are: Atorvastatin, Metformin, Doxycycline and Mebendazole.
The following figure briefly illustrates the synergistic effect of these drugs.
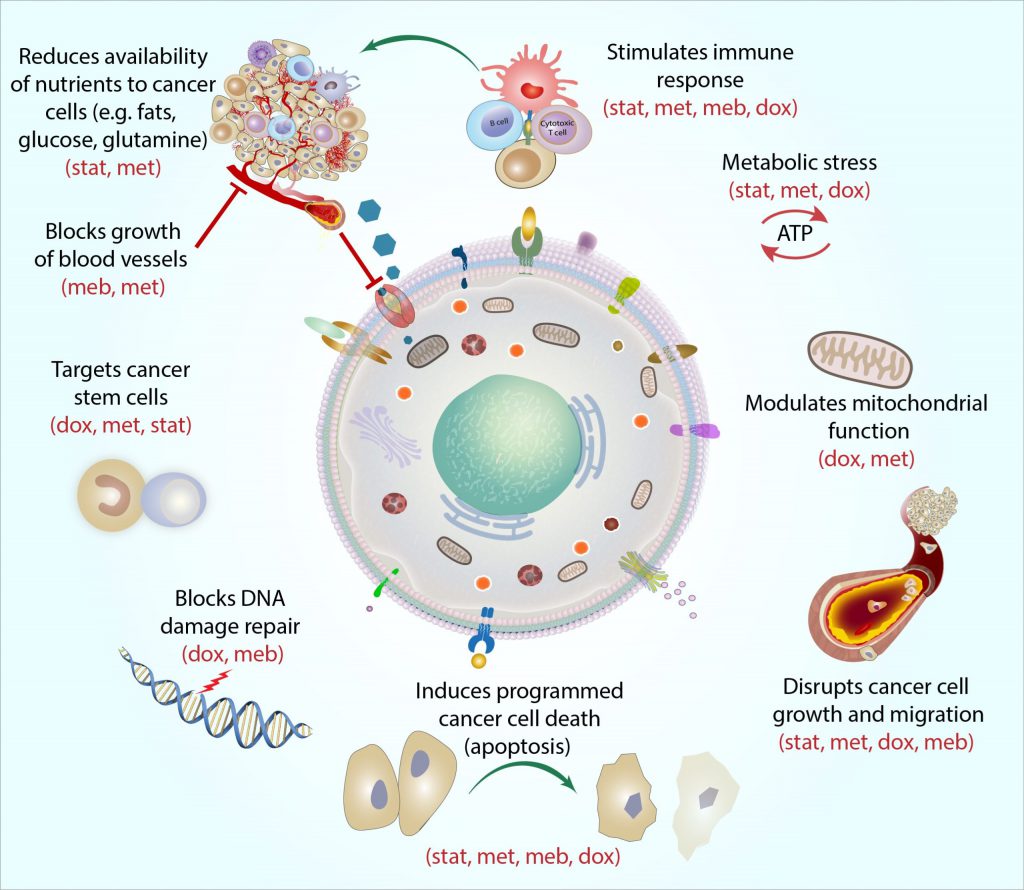
In addition to the data referring to the individual drugs used in the Care Metabolic Protocol ™, a statistically significant result was achieved in a clinical study of 100 patients on GBM WHO grade IV, registered as a METRICS study on clinicaltrials.gov. According to this study, two-year survival increases from 26.5% with the standard protocol of care (surgery, radiation and temozolomide) to 60% when the Care protocol is used in addition to the standard protocol.
| Variable considered | Palliative Care | Standard-of-care ** | Standard-of-care + COC Protocol * |
| Median survival from diagnosis | 3,0 months | 14,8 months | 26,9 months |
| 2-year overall survival | 6,6% | 26,5% | 60,0% |
** Data from 2015 Public Health England
Regarding safety, metformin and atorvastatin are two of the most prescribed drugs in the world. Doxycycline came into commercial use in 1967 and was prescribed to children and adolescents for acne for prolonged periods. Mebendazole, a very safe drug, entered clinical use in 1971 as an anthelmintic and is now sold over the counter in many countries. Furthermore, this likely increase in overall survival is not at the expense of quality of life.
Metabolic dysregulation (Warburg effect) is one of the oldest known properties of the cancer cell and is an exploitable vulnerability. Metabolically targeted therapies are ideal as add-on therapies to cytotoxic agents, immunotherapies and targeted therapies for a variety of reasons: they weaken the cancer cell so that it is easier to kill and, they increase the internal and external mechanisms that facilitate apoptosis, thus mitigating the death of necrotic cells, the burden of tumor lysis and inflammation. The Care Metabolic Protocol ™ exerts its additional therapeutic effect in general by targeting the dysregulated consumption of glucose by cancer cells (20 times that of a normal cell), the epigenetic upregulation of apoptotic factors, the downregulation of transcription oncogenes, stimulation of the immune system and other mechanisms. In summary, the use of the Care Metabolic ™ protocol seems to have several advantages:
- Quality of Life: The protocol includes repurposed drugs with minimal side effects.
- Safety: consolidated knowledge of dosage, pharmacokinetics, toxicity and interactions from long-term clinical use.
- Aims at ubiquitous metabolic dysregulation: ideal for adjunct therapy.
- It provides a treatment option to prevent / delay relapses.
If you’ve come this far, I hope I’ve provided you with some useful information. However, I recommend that you talk to the team of doctors who are following you or who are following your loved ones since the opportunity to use an adjuvant protocol of this type depends on the specific case, i.e. on the individual patient and their specific health conditions.
My wife diagnosed with front left temporal GBM stage 4 in August 2018 ,had 13 hour debulking followed by a shunt insertion 1 month radiotherapy,TMZ etc then MRI
July 2020 showed disease progression
after much messing about (because of the part of the world we’re in)Lomustinee,procarbazine, vincristine were eventually prescribed in November 2020 But by this time I’d just started giving my wife Simvastatin And Mebendazole that I’d bought over the counter from the local pharmacy but because I’d only limited information about dosing it was hit and miss how I’ve been giving them (statins between 20mg -80mg per day divided in to 3-4 doses,Mebendazole 1000mg-2000mg a day divided 3-4 doses), another MRI in July 2021 revealed that the original front left tumor site was now cancer free But a new tumour has appeared on the Right side, the hospital want to do another surgery but my wife don’t want and is not wanting it,any suggestions from anyone on dosage of Mebendazole,Statins would be appreciated as I’m wondering if increasing the dose of MB to 4000mg a day divided into 4 is to much/little?? One more thing,of all the doctors my wife has seen Not a single one when she was first diagnosed said she would live past August 2019.
P.s I read that fluoxotine induced cancer cell death in GBM so I’ve had her taking 20 -40mg a day for the last couple of months, any info on anything would be appreciated,
Thanks.
I hope your wife is doing better, now. I have had Glioblastoma brain cancer since last August.
~Linda
How did you get cured? I’ve Just passed through surgery and didn’t start treatment yet. Thanks, God bless you.