Some Points from the Final Conference of the TRANS-GLIOMA Project and the Idea of the Glioblastoma Navigator
I apologize for having reduced the frequency of publication of new articles but as you know we are trying to transform glioblastoma.it into a voluntary organization. In any case, here we are. As you know, some time ago I was sent to the final conference of the Trans-Glioma project with the patients’ point of view derived from the experience and analytical data of the use of this site.
I got some interesting information from the conference. For example, it seems that neurosurgery has reached a considerable level of efficiency and at least in the centers that can operate patients by keeping them in a state of wakefulness and being able to test the responses to stimuli it is really able to minimize collateral damage. Now it is possible to operate tumors that were once inoperable, removing as many visible tumoral tissue also thanks to 5-ALA which allows to distinguish tumor cells from healthy cells. Some tumors remain, especially if their location is deep or close to critical functional areas or with elevators that are at risk of bleeding.
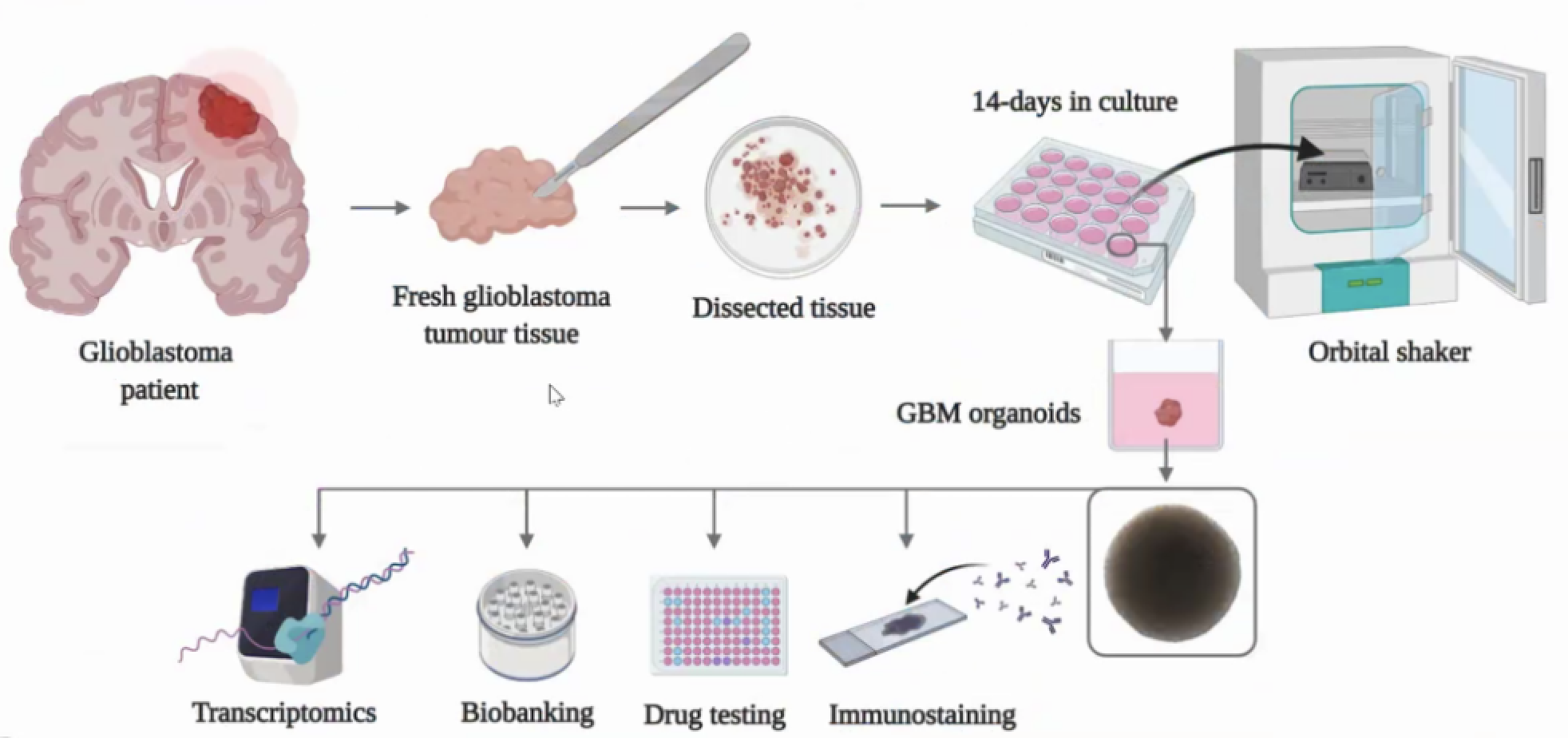
Another interesting part is certainly the state of the development in the field of organoids description that can be extremely useful for more targeted and personalized therapies and to evaluate in advance the possible outcomes of translational therapies. The following figure illustrates how it works.
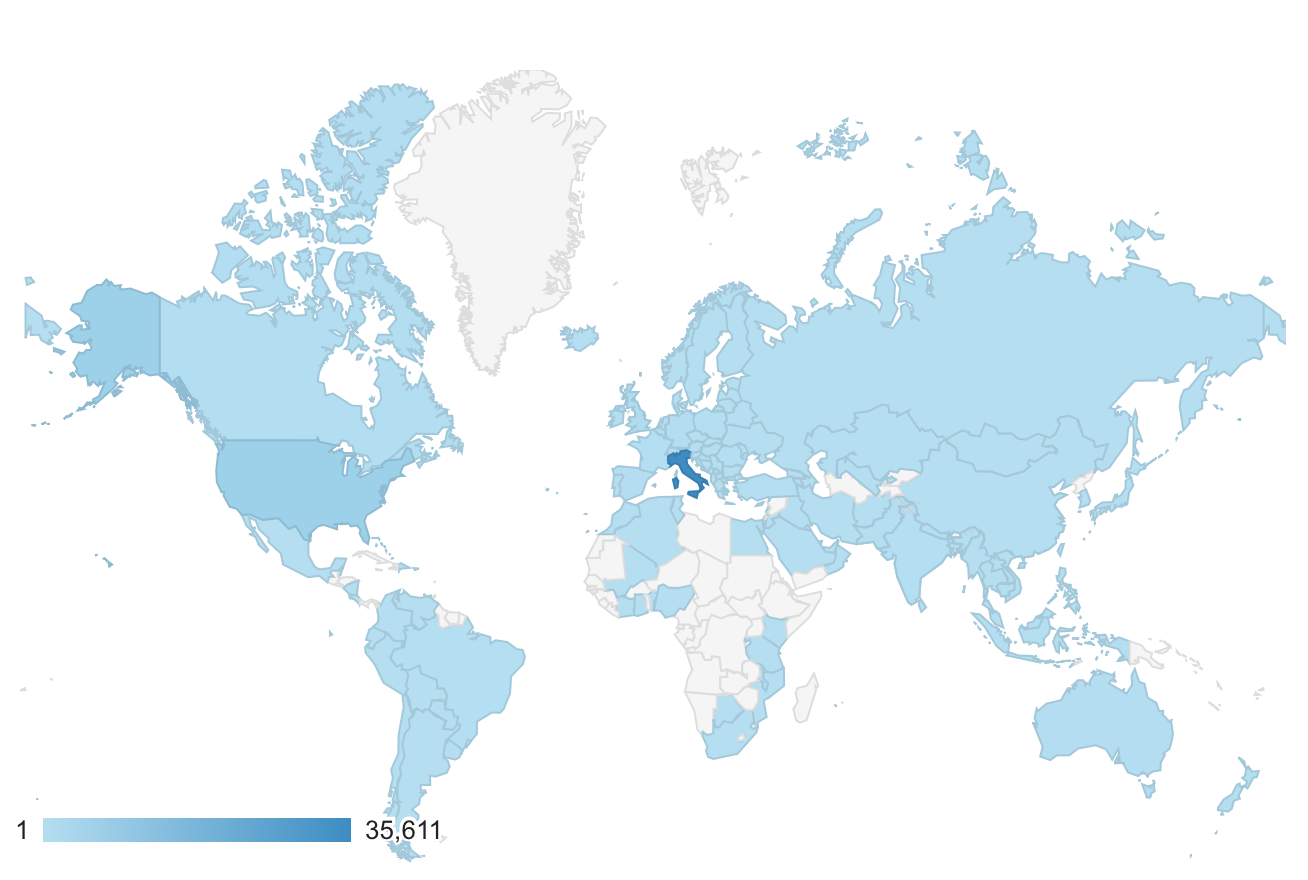
During the conference we were also asked to talk about the glioblastoma.it website and the needs of patients and their families and caregivers. The site currently has almost 50,000 visitors of which 3 out of 4 come from Italy but it is possible to see from the figure below there are visitors from all over the world.
The idea of the Glioblastoma Navigator database was also presented, which I summarize below. The Virtual Trial Database is only available in English and its new version is not accessible by Italian patients or patients not residing in the United States and free of charge. The idea of developing the Glioblastoma Navigator database stems from the need to overcome the limits of the Virtual Trial Database, making it easily accessible even to patients not resident in the United States. The name comes from a metaphor borrowed from robotics and in particular from the problem of autonomous navigation: to navigate you need a reference map, know where you are located and where you want to go and plan a route (global navigation) and proceed avoiding obstacles (local navigation). We will start by gathering information about patients (their residence) and present information about treatment centers by allowing patients to offer feedback on their experiences. Geographic information could be analyzed and compared with other open access data with geographic information (for example data on some environmental factors) to be able to obtain useful information for policy makers (global navigation). We could also provide patients with the closest centers with the best feedback (local navigation). We may also gradually collect information on the specific lesion, mutations, markers, integrated treatments and responses to treatments in order to be able to obtain useful information through the application of artificial intelligence and machine learning methods. This knowledge can still be made easily accessible through gliobots as well.
Clinical trials are slow and many, especially those not directly funded by pharmaceutical companies because for example they use an approach that combines different treatments, are struggling to obtain the funds. The CUSP-ND campaign: trial to beat Glioblastoma in memory of Emanuele also goes on really slowly. The time needed to move from the idea to the different phases of the clinical trial, obtain the results and publish them and make them available to patients is 5-10 years! And we often only talk about approved treatments. Data and results on off-label or repositioned supplements and drugs are even more difficult to obtain. That’s why in my opinion there is a need for a project of this type.
I would like to complete the development of this project by the end of the year. Development will be conducted iteratively with periodic releases of versions with new features starting in the next few months. Patient data will be collected anonymously (i.e. through a code not linked to the patient’s identity) and a quality control will be applied to it.
I would like to receive your comments on this idea. See you soon!